Rational design of affinity-based peptide carriers
The transport and binding behavior of solutes inside tissues is a complex function of a wide range of parameters. This project focuses on determining the rules of combination of size, net electric charge and its spatial distribution, hydrophobicity, and effects of other binding interactions to design polypeptide-based carriers for targeting tissues of varying fixed charge density. We use both experiments and theoretical transport models to rationally design nanocarriers for delivery of wide range of biological entities including small molecules, protein growth factors, antibodies and gene materials that can penetrate through the dense negatively charged tissue matrix barriers (eg., cartilage, meniscus, intervertebral discs, mucin, eyes) and reach their target site.
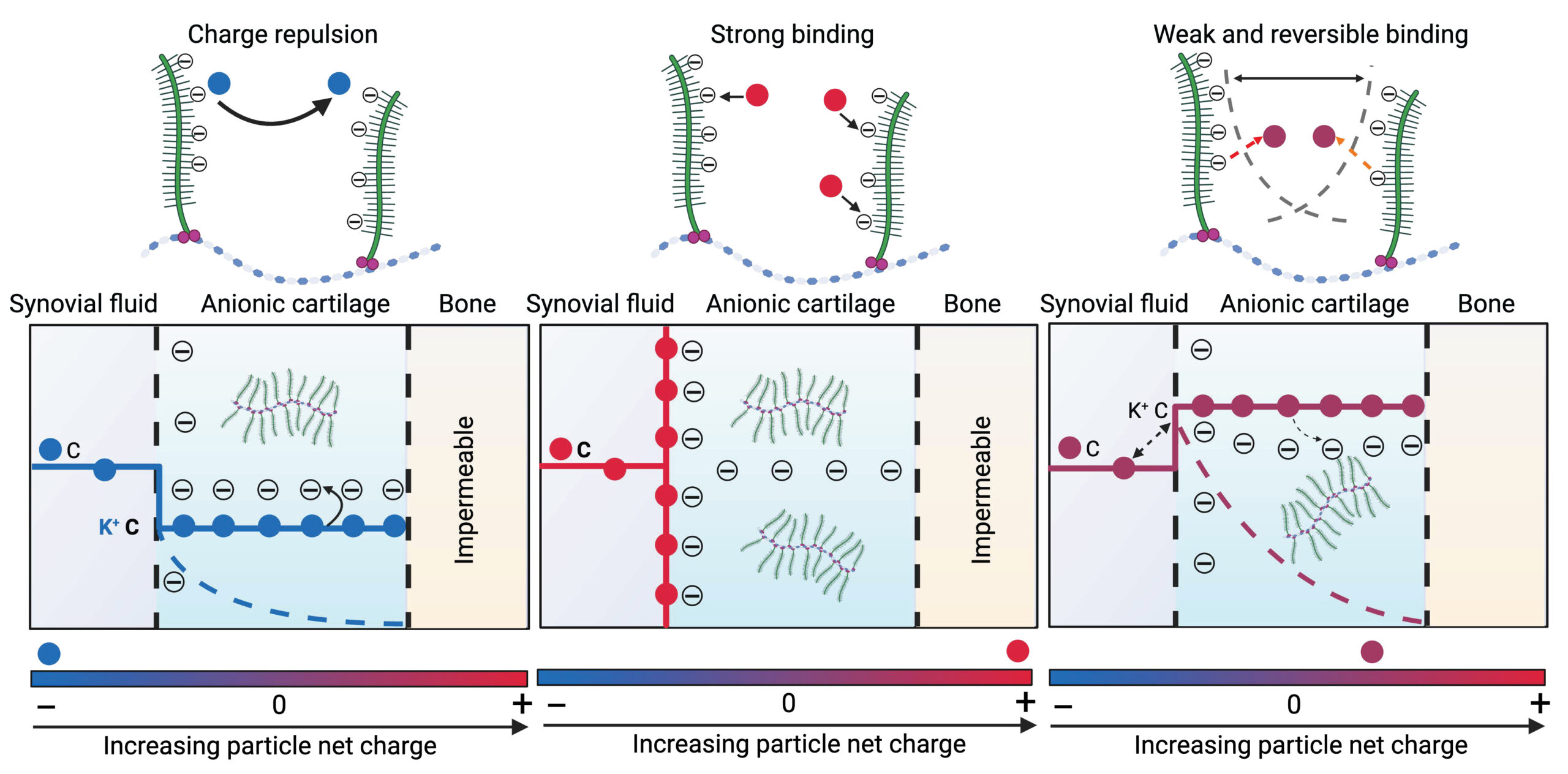
We are also interested in understanding the downstream effects of large MW, highly cationic nanocarriers on the electromechanochemical and biological homeostasis of tissues. It is critical to probe how very high intra-tissue uptake of such charged carriers may induce mechanistic changes to aid rational design of charged drug delivery vehicles for clinical translatability.
Representative Publications
- Charge-based drug delivery to cartilage: Hydrophobic and not electrostatic interactions are the dominant cause of competitive binding of cationic carriers in synovial fluid. Acta Biomaterialia, 2022 Read
- Effects of polycationic drug carriers on the electromechanical and swelling properties of cartilage. Biophysical Journal, 2022 Read
- Cationic peptide carriers enable long-term delivery of insulin-like growth factor-1 to suppress osteoarthritis-induced matrix degradation. Arthritis Research & Therapy, 2022 Read
- Cartilage penetrating cationic peptide carriers for applications in drug delivery to avascular negatively charged tissues. Acta Biomaterialia, 2019 Read
- Overcoming negatively charged tissue barriers: Drug delivery using cationic peptides and proteins. Nano Today, 2020 Read
Research Talks
Osmotic swelling in response to cationic peptide carrier treatment. Warren et al, SB3C 2020
Cationic Peptide Carriers for cartilage targeting. Vedadghavami et al, SB3C 2020
Cationic multi-arm Avidin – a drug delivery platform carrier
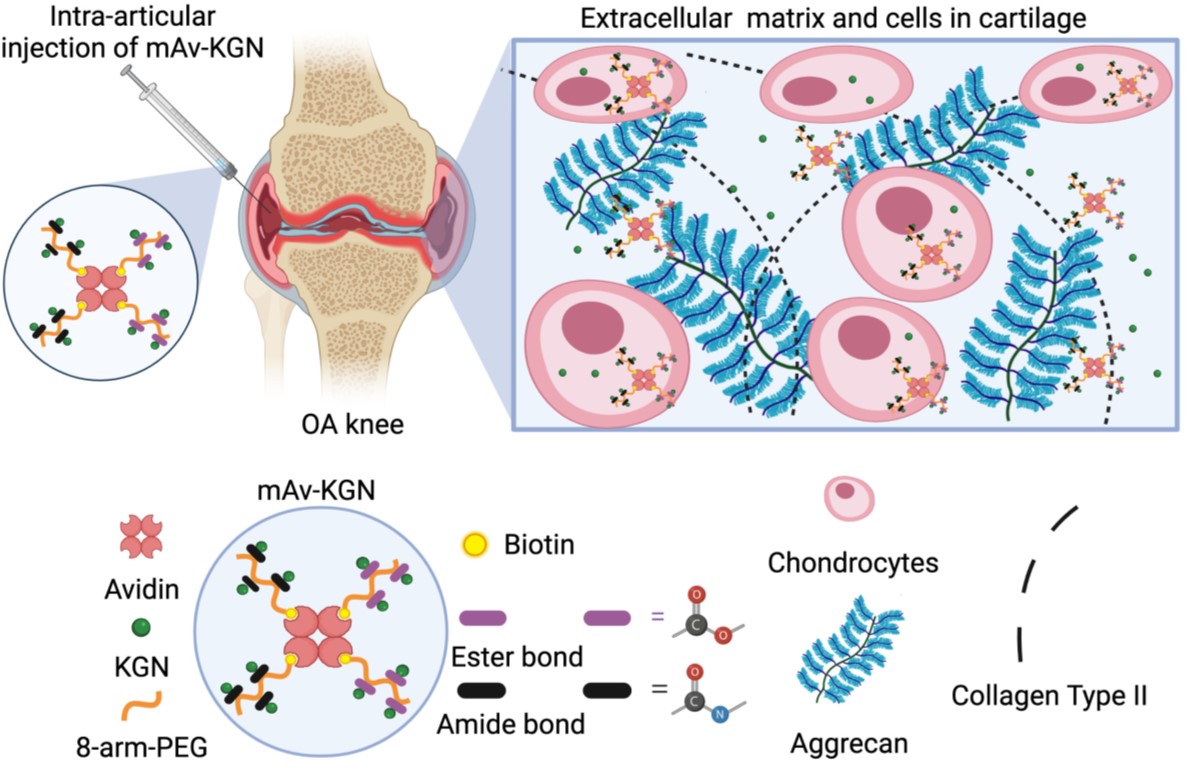
Targeted drug delivery to joint tissues like cartilage remains a challenge that has prevented clinical translation of promising osteoarthritis (OA) drugs. Local intra-articular (IA) injections of drugs suffer from rapid clearance from the joint space and slow diffusive transport through the dense, avascular cartilage matrix comprised of negatively charged GAGs. We have designed a multi-arm cationic nano-construct of Avidin (mAv) that can be covalently conjugated with small molecule drugs using hydrolysable linkers. The construct can rapidly penetrate through the full thickness of cartilage in high concentration and have long intra-cartilage residence time in both healthy and arthritic cartilage via weak-reversible binding with negatively charged aggrecans. The carrier when conjugated to small molecule OA drugs can effectively suppress OA associated catabolic effects and enable regeneration with a single low dose.
This charge-based cartilage homing drug delivery platform can elicit disease modifying effects as well as facilitate long-term symptomatic pain and inflammation relief by enhancing tissue specificity and prolonging intra-cartilage drug residence time.
Representative Publications
- Intra-articular kinetics of a cartilage targeting cationic PEGylated protein for applications in drug delivery. Osteoarthritis and Cartilage, 2022 Read
- Single-Dose Intra-Cartilage Delivery of Kartogenin Using a Cationic Multi-Arm Avidin Nanocarrier Suppresses Cytokine-Induced Osteoarthritis-Related Catabolism. Cartilage, 2022 Read
- Avidin grafted dextran nanostructure enables a month-long intra-discal retention. Nature Scientific Reports, 2020 Read
- Multi-arm Avidin nano-construct for intra-cartilage delivery of small molecule drugs. Journal of Controlled Release, 2019 Read
- Cartilage Targeting Drug Delivery: Can Electrostatic Interactions Help? Nature Rheumatology Reviews, 2017 Read
Research Talks
Delivery of dexamethasone using multi-arm avidin. He et al, SB3C 2020
Charge-reversed tissue targeting cationic exosomes
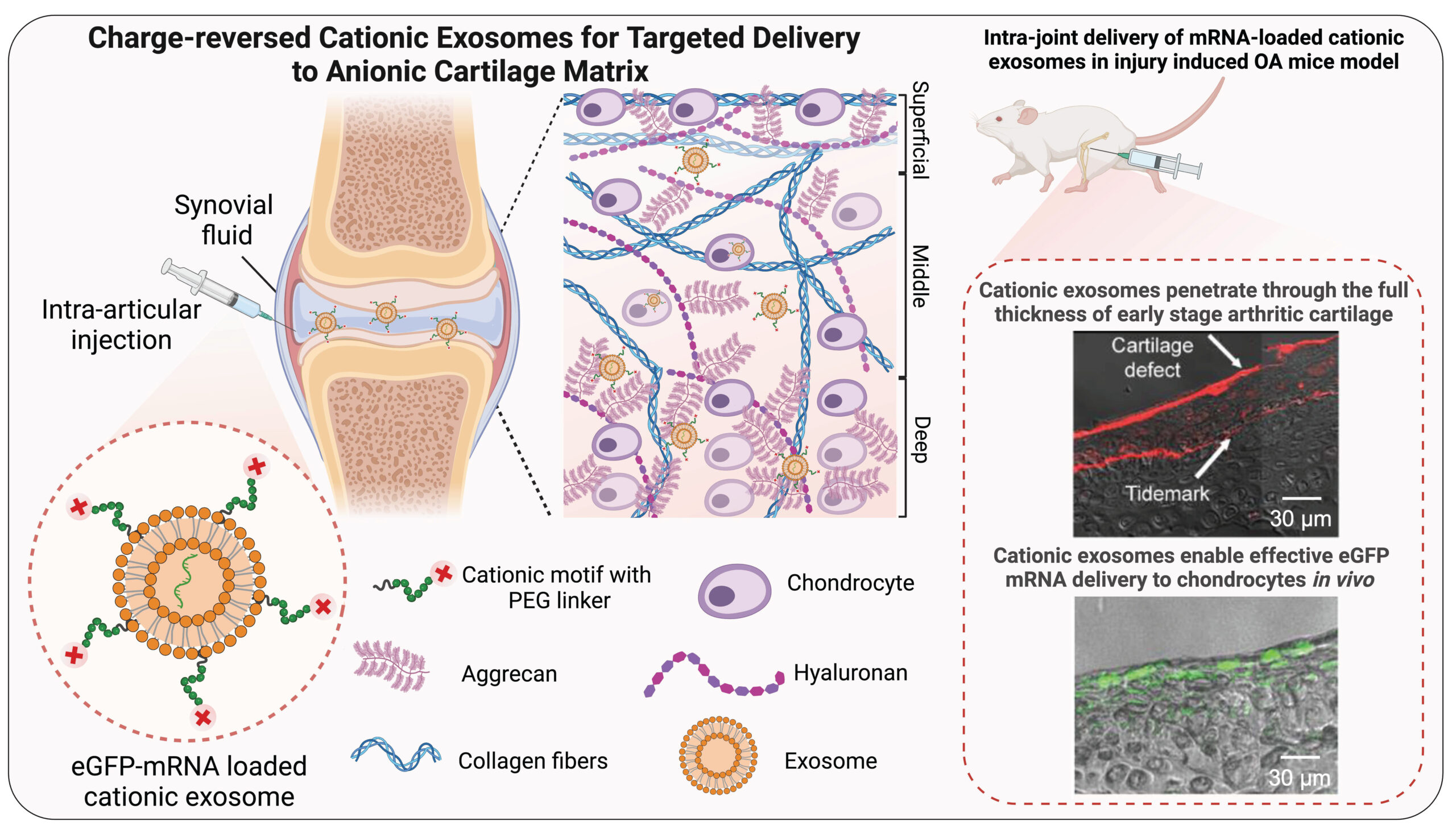
Cationic exosomes for cartilage repair: MSC exosomes are emerging as promising therapeutics for OA. The negative charge of exosome lipid bilayer, however, hinders their penetration into the negatively charged cartilage. To enhance their intrinsic therapeutic potential, we have designed a new class of charge-reverse cartilage targeting cationic exosomes that can penetrate through the full thickness of early-stage arthritic cartilage and can deliver the encapsulated gene or protein therapeutics. Cationic exosomes hold strong translational potential as a platform technology for cartilage-targeted non-viral delivery of any relevant mRNA targets for OA treatment.
Mucus penetrating milk exosomes for oral drug delivery: We have engineered high purity milk exosomes (mExo) with modular surface tunability for oral delivery of siRNA by utilizing a low-cost enrichment method combining casein chelation with differential ultracentrifugation. PEGylated mExo anchored with zwitterionic motifs can penetrate through the mucin to target intestinal epithelial cells and deliver packed genes. We have incorporated different functional reagents on mExo surface by click chemistry enabling tunable properties.
Charged probes for diagnostic imaging of cartilage
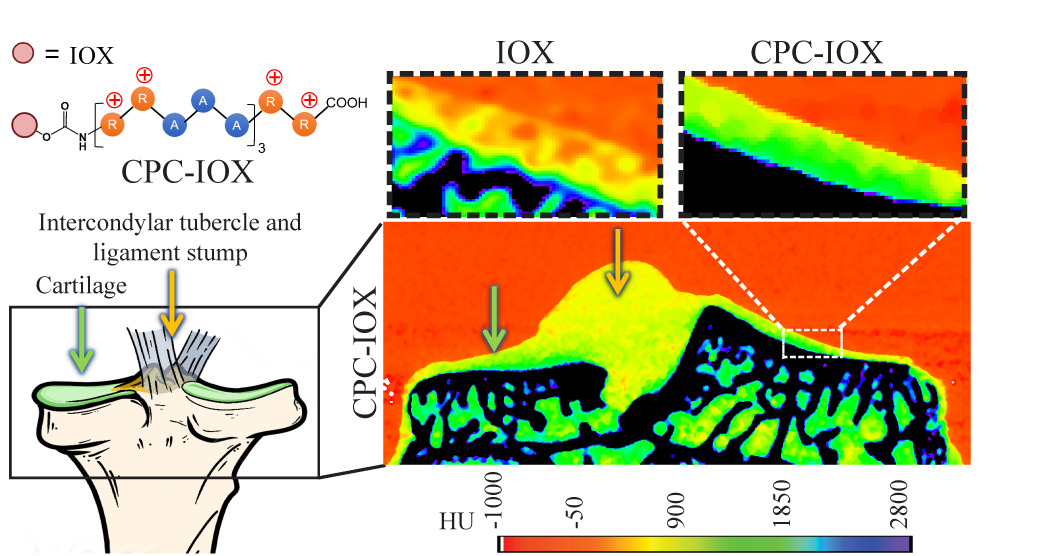
Early OA diagnosis is critical as there is a narrow time-window for therapeutic intervention. Computed tomography (CT) of cartilage is not clinically viable, which exhibits early OA changes. We have synthesized optimally charged cationic contrast agents for cartilage imaging that can rapidly penetrate through full cartilage thickness in high concentrations using weak-reversible electrostatic interactions. These cationic contrast agents demonstrate high CT signal at 160x lower doses of IOX, thereby significantly minimizing side effects associated with high dose IOX. The CT signal correlates strongly with spatial GAG distribution, which can enable diagnosis of early-stage OA.
Representative Publications
- Cationic Carrier Mediated Delivery of Anionic Contrast Agents in Low Doses Enable Enhanced Computed Tomography Imaging of Cartilage for Early Osteoarthritis Diagnosis. ACS Nano, 2023 Read
- Charge driven contrast enhanced computed tomography for imaging of negatively charged tissues. Book Chapter, Methods in Molecular Biology, 3rd Edition, Humana Press (Springer Science), 2022 Read
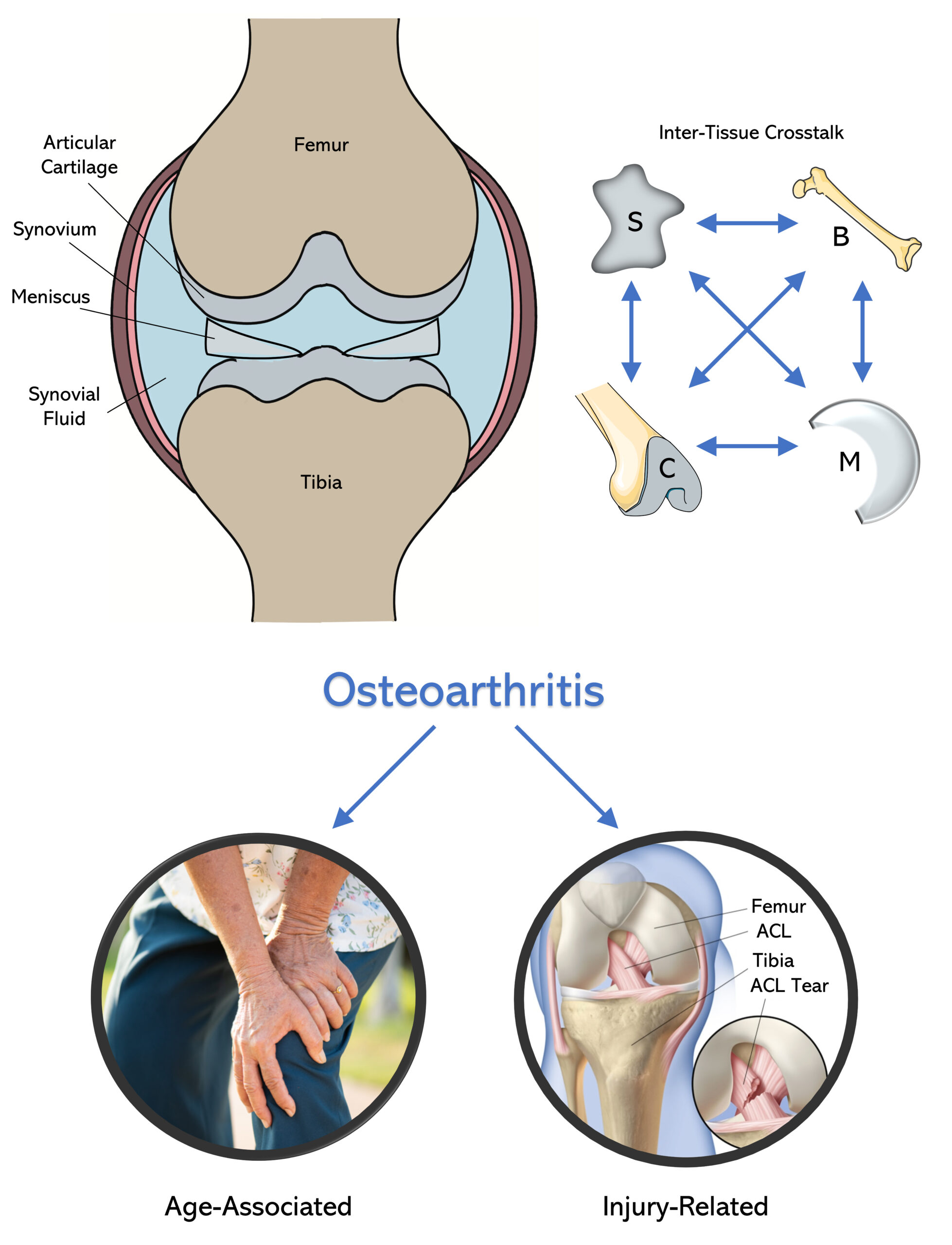
Biological models for post-traumatic & aging induced osteoarthritis
In the case of traumatic joint injury (i.e. ACL or meniscus tears), cartilage and synovium tissues engage in significant biological crosstalk with each other, thereby accelerating the rate of OA development (10-20 years). Upon injury, mechanical trauma causes cartilage debris to travel and trigger inflammation of the synovial lining layer. As a response, the synovium releases several pro-inflammatory cytokines (i.e. IL-1, TNFα) and degradative enzymes (i.e. MMP3, Aggrecanases) which further break down cartilage matrix proteins, thus creating a vicious cycle of inflammation and degradation. This project focuses on developing in vitro co-culture models of PTOA that include a combination of cartilage, synovium, meniscus and bone for achieving an improved understanding of inter-tissue crosstalk and for evaluating the biological efficacy of potential disease modifying therapies.
In the case of aging induced OA, cartilage is significantly impacted by both mechanical crosslinking and biochemical changes. As an individual ages, accumulated sugars within cartilage begin to react non-enzymatically with collagen II amine groups to form irreversible crosslinks known as advanced glycation endproducts (AGEs). As a result, the cartilage matrix becomes increasingly stiff and brittle, thereby making it more susceptible to mechanical degradation. AGEs also have the potential to bind to cellular receptors present on both chondrocytes and synoviocytes, which can lead to increase in inflammatory and senescent activity coupled with decrease in cellular metabolism and matrix production. This project evaluates the response to anti-aging drugs in an effort to prevent aging and diabetes induced OA.
Representative Publications
- Resveratrol and Curcumin attenuate ex vivo sugar-induced cartilage glycation, stiffening, senescence and degradation. Cartilage, 2021 Read
- Interleukin-1 receptor antagonist (IL-1Ra) is more effective in suppressing cytokine induced catabolism in cartilage-synovium co-culture than cartilage monoculture. Arthritis Research Therapy, 2019 Read
Research Talks
Aging induced OA. Mehta et al, SB3C 2020
